Tag: pharmaceutical
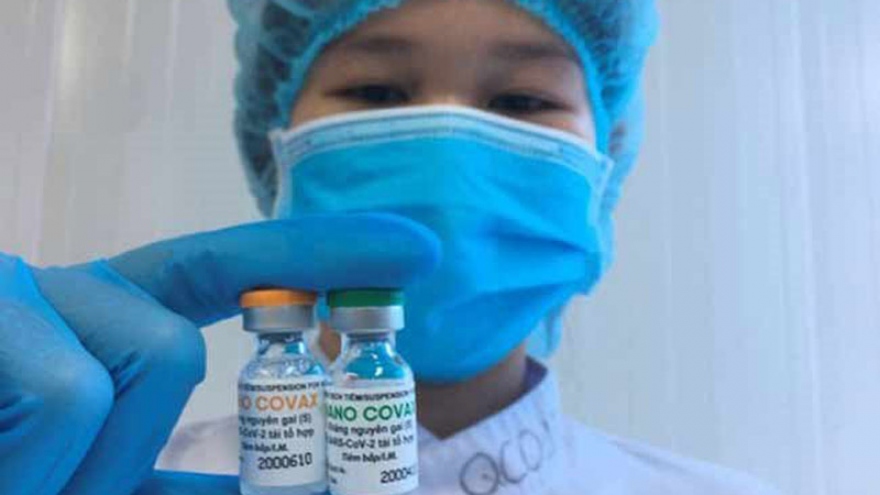
RoK firm to provide Vietnam’s Nano Covax vaccine worldwide
Korean firm HLB has signed a memorandum of understanding with Nanogen Pharmaceutical Biotechnology JSC, producer of Vietnam's Nano Covax COVID-19 vaccine candidate, to acquire the rights to distribute the vaccine worldwide, except in Vietnam and India, when the vaccine is approved for use.

Indian company partners with Nanogen to distribute Nano Covax vaccine
VOV.VN - Indian firm Vekaria Healthcare LLP and Nanogen Pharmaceutical Biotechnology JSC, the Vietnamese developer of Nano Covax vaccine against COVID-19 have signed a non-disclosure agreement (NDA) on the transfer of technology to produce the vaccine.

First FDA-licensed Remdesivir doses arrive in Vietnam
VOV.VN - Approximately 10,000 vials of Remdesivir, a broad-spectrum antiviral drug licensed by the US Food and Drug Administration (FDA) for COVID-19 treatment arrived in Vietnam on August 5 evening.

India to deliver 1 mln COVID-19 Remdesivir doses to Vietnam
VOV.VN - Some of big Indian pharmaceutical companies have agreed to provide Vietnam with approximately 1 million doses of Remdesivir, a broad-spectrum antiviral medication, for COVID-19 treatment.
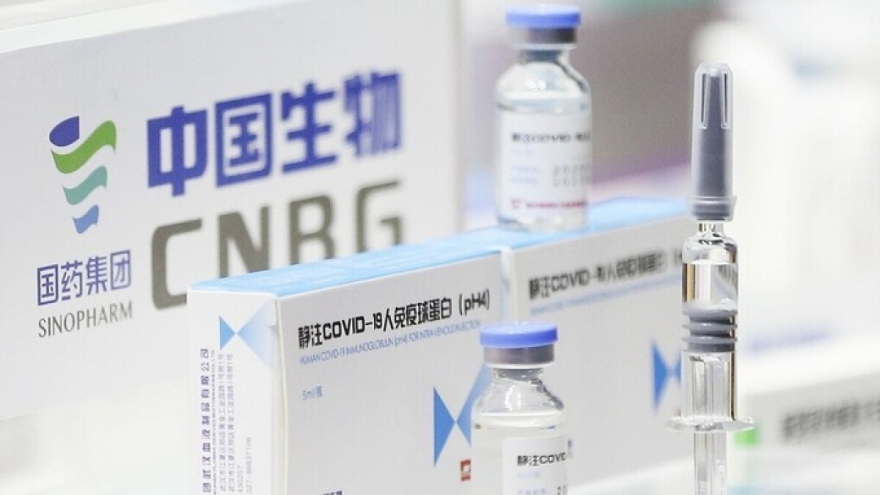
One million Sinopharm vaccine doses arrive in HCM City
VOV.VN - One million doses of Vero Cell vaccine arrived in Ho Chi Minh City on July 31 as part of a deal to import five million doses of the vaccine from Chinese pharmaceutical company Sinopharm.

Indian pharma group has eyes on Da Nang
A group of 30 pharmaceutical production companies from India plans to build the first Pharma Park in Vietnam, and Da Nang city’s Hi-tech Park has been suggested as a prime location.
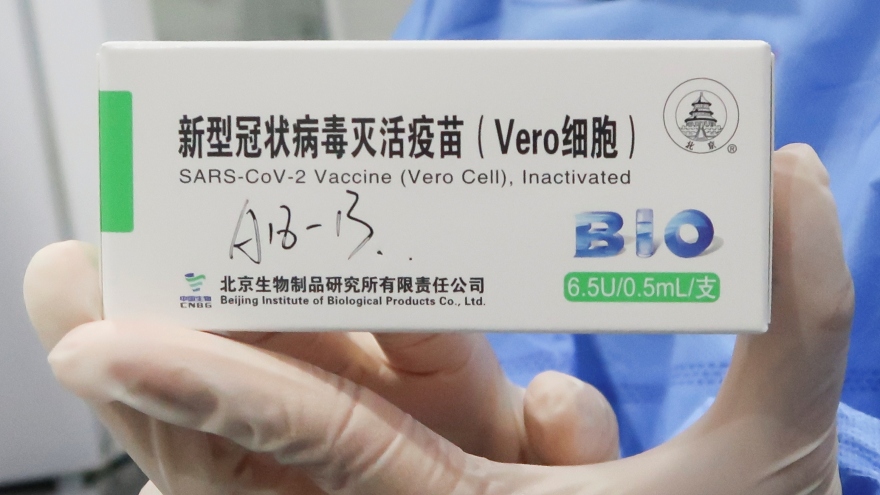
HCM City pharmacy firm imports 5 million doses of Sinopharm vaccine
VOV.VN - The Ministry of Health has permitted a pharmaceutical company of Ho Chi Minh City to purchase five million doses of China’s Sinopharm COVID-19 vaccine known as Vero Cell for emergency use.

219 new COVID-19 cases detected as daily infection count climbs to 545
VOV.VN - A further 219 people, including 9 entrants to the country, tested positive for the SASR-CoV-2 virus on the evening of July 2, thereby lifting the daily infection count to 545, according to figures given by the Ministry of Health.

Three localities report 43 community infections over 12 hours
VOV.VN - A further 43 local COVID-19 cases and one imported infection were recorded on June 8 morning, with current hotspots of Bac Ninh, Bac Giang and Ho Chi Minh City taking the most, according to data released by the Health Ministry.
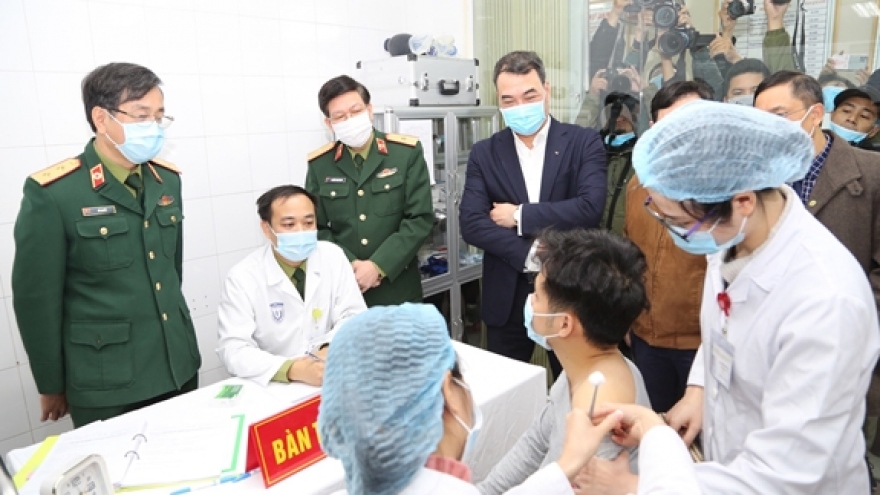
Third phase of Nano Covax human trials due to begin in May
VOV.VN - Nanogen Pharmaceutical Biotechnology JSC have announced plans to launch the third phase of human trials for its locally-produced coronavirus vaccine Nano Covax on May 5, a timeframe which is three months ahead of schedule.









