Tag: nanocovax
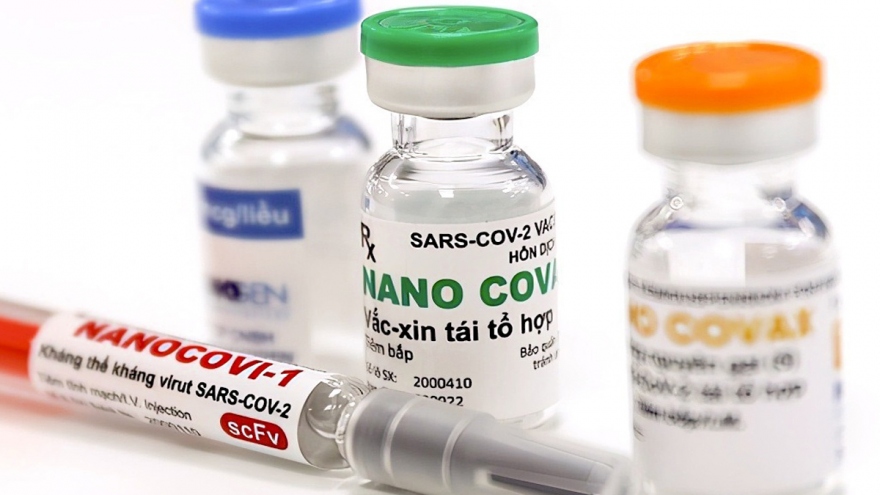
Vietnam considers licensing locally made Nanocovax COVID-19 vaccine
VOV.VN - Deputy Prime Minister Vu Duc Dam has requested the Ministry of Health to consider issuing a registration certificate for domestic circulation of homegrown Nanocovax vaccine against COVID-19.

Nanocovax approved by National Biomedical Research Ethics Council
VOV.VN - Results from the phase 3a clinical trial of the domestically-produced COVID-19 vaccine Nanocovax on more than 1,000 volunteers have been adopted by the National Biomedical Research Ethics Council (NBREC).

Indian company partners with Nanogen to distribute Nano Covax vaccine
VOV.VN - Indian firm Vekaria Healthcare LLP and Nanogen Pharmaceutical Biotechnology JSC, the Vietnamese developer of Nano Covax vaccine against COVID-19 have signed a non-disclosure agreement (NDA) on the transfer of technology to produce the vaccine.
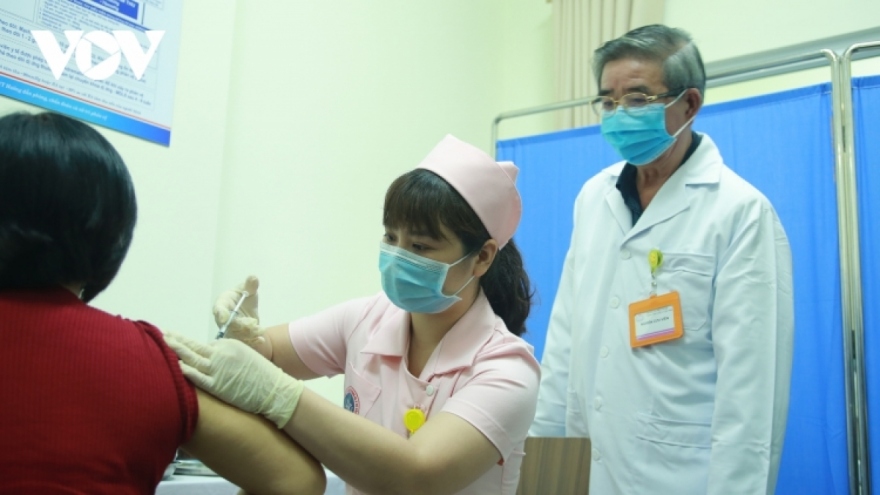
Local COVIVAC vaccine to enter second phase of clinical trials
VOV.VN - The National Institute of Hygiene and Epidemiology is set to recruit the first group of volunteers on August 10 for the second phase of clinical trials of the locally-produced COVIVAC vaccine in the northern province of Thai Binh.

Vietnam poised to start clinical trials of third COVID-19 vaccine this month
VOV.VN - The Ministry of Health has recently granted approval for clinical trials to begin on the ARCT-154 COVID-19 vaccine, with medical tests set to begin on August 8 at Hanoi Medical University.

Vietnam strives for at least one successful homegrown COVID-19 vaccine in 2021
Vietnam strives to have at least one successful domestically developed COVID-19 vaccine this year, according to deputy health minister Tran Van Thuan.
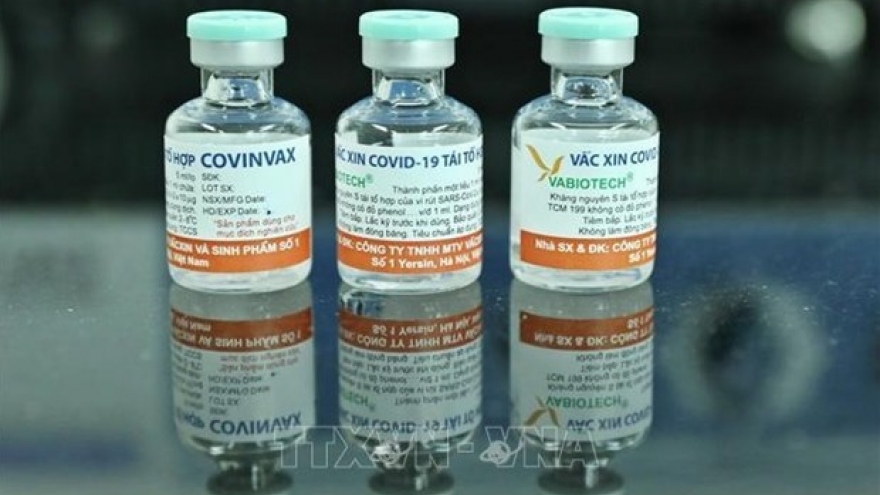
Vietnam has COVID-19 vaccine production capacity: Officer
Vietnam can satisfy COVID-19 vaccine production requirements once it obtains vaccine patents, an officer has affirmed.
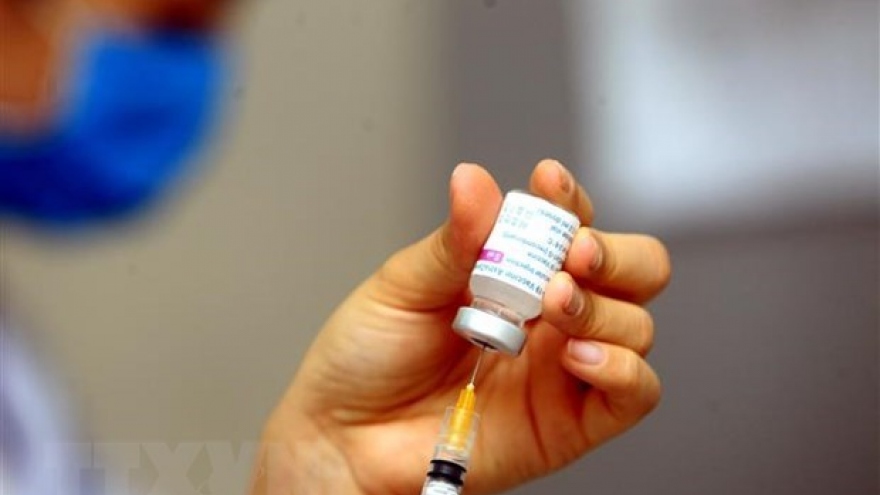
Vietnam's COVID-19 vaccine set to begin phase 3 trials in June
Vietnam’s domestically developed COVID-19 vaccine, Nano Covax, will begin Phase 3 of clinical trials in June involving up to 13,000 volunteers.
Vietnam likely to produce first homegrown COVID-19 vaccines this year
VOV.VN - Vietnam is hopeful it can produce its first local COVID-19 vaccine this year, with the third trial phase of trials for the NanoCovax vaccine set to be completed in late September while the COVIVAC vaccine will enter the second phase by the end of June, according to the Ministry of Health (MoH).
Second phase of Nano Covax human trials set for completion on Apr.8
VOV.VN - Vietnam is scheduled to finalize the second phase of human trials for NanoCovax, a local-produced novel coronavirus (COVID-19) vaccine, on April 8.









