Tag: NANOGEN
Vietnam considers licensing locally made Nanocovax COVID-19 vaccine
VOV.VN - Deputy Prime Minister Vu Duc Dam has requested the Ministry of Health to consider issuing a registration certificate for domestic circulation of homegrown Nanocovax vaccine against COVID-19.
Health professionals okay homegrown vaccine Nano Covax
VOV.VN - The National Committee for Ethics in Biomedical Research under the Ministry of Health (MoH) on December 29 voted in favour of the results of the third human clinical trial of locally produced vaccine Nano Covax against the SARS-CoV-2 virus.
India to evaluate Vietnam’s Nano Covax COVID-19 vaccine quality
VOV.VN - India’s Translational Health Science and Technology Institute (THSTI) will test Vietnam’s Nano Covax COVID-19 vaccine developed by Nanogen Pharmaceutical Biotechnology JSC to see if it meets standards for use.
Vietnam to have at least one homegrown COVID-19 vaccine by year-end
VOV.VN - By the end of this year Vietnam will have at least one locally-produced COVID-19 vaccine which will be licensed and inoculated for local people from the start of early 2022, according to the Ministry of Health (MoH).

Further evaluation needed for home-grown vaccine Nano Covax: Deputy Minister
Nanogen Pharmaceutical Biotechnology JSC was asked to provide more information regarding safety, immunogenicity, and protection effects before a registration certificate of circulation for its Nano Covax can be granted, Deputy Minister of Health Tran Van Thuan has said.

Vietnam yet to approve homegrown Nano Covax vaccine
VOV.VN - The Advisory Council for the Registration of Circulation of Drugs and Medicinal Ingredients on August 29 did not approve Nano Covax, the first locally produced COVID-19 vaccine, and requested additional data to ensure the vaccine is completely safe and effective.

Nanocovax approved by National Biomedical Research Ethics Council
VOV.VN - Results from the phase 3a clinical trial of the domestically-produced COVID-19 vaccine Nanocovax on more than 1,000 volunteers have been adopted by the National Biomedical Research Ethics Council (NBREC).
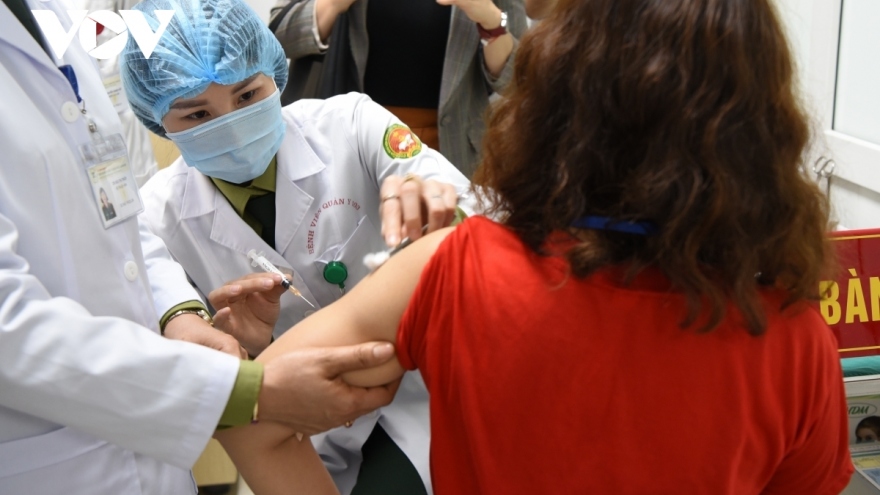
Promising results for Nano Covax vaccine in phase 3a of clinical trials
VOV.VN - A local research team working on developing Nano Covax, a homegrown COVID-19 vaccine, have unveiled the promising outcomes of the mid-term phase 3a clinical trials.
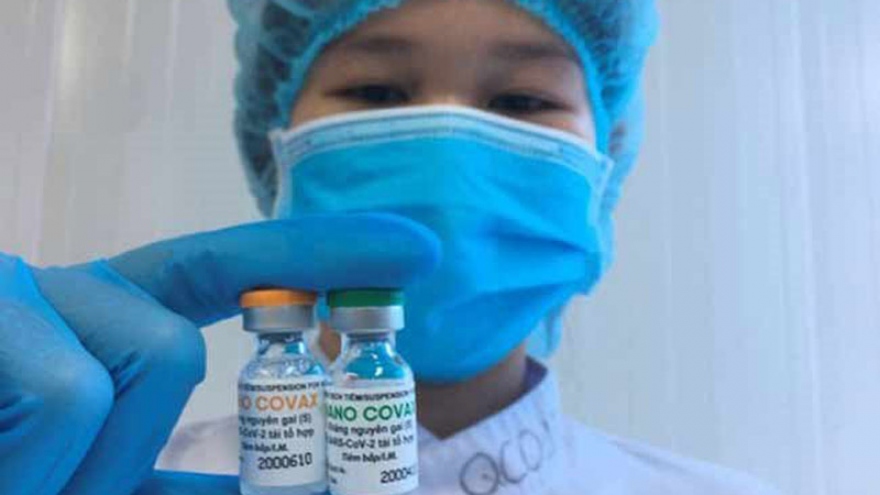
RoK firm to provide Vietnam’s Nano Covax vaccine worldwide
Korean firm HLB has signed a memorandum of understanding with Nanogen Pharmaceutical Biotechnology JSC, producer of Vietnam's Nano Covax COVID-19 vaccine candidate, to acquire the rights to distribute the vaccine worldwide, except in Vietnam and India, when the vaccine is approved for use.

Indian company partners with Nanogen to distribute Nano Covax vaccine
VOV.VN - Indian firm Vekaria Healthcare LLP and Nanogen Pharmaceutical Biotechnology JSC, the Vietnamese developer of Nano Covax vaccine against COVID-19 have signed a non-disclosure agreement (NDA) on the transfer of technology to produce the vaccine.
