Tag: COVIVAC
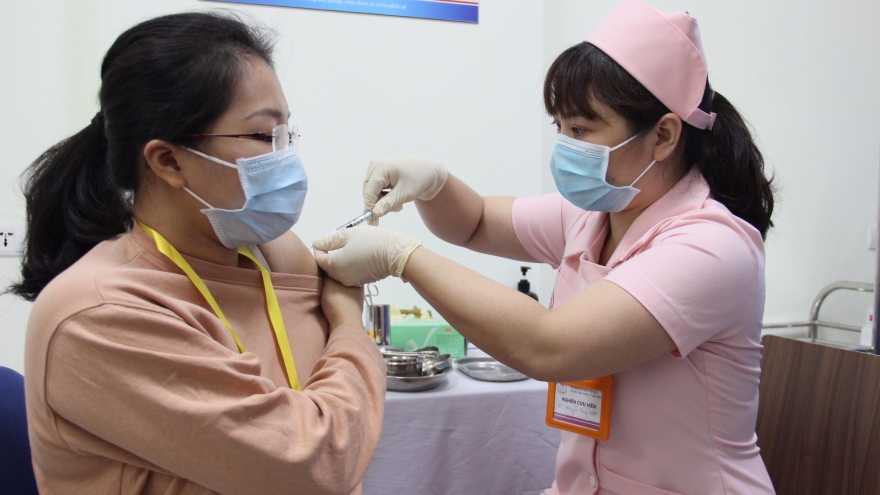
Six volunteers stable after first shot of second local COVID-19 vaccine
VOV.VN - The initial six volunteers participating in human trials of Covivac, the second locally-produced COVID-19 vaccine, remain in stable condition after receiving a dose of the experimental vaccine on March 15.

Initial phase of clinical trials on Covivac begins on March 15
VOV.VN - The Hanoi Medical University is to start the initial phase of clinical trials on the second domestically-produced COVID-19 vaccine known as Covivac, developed by the Institute of Vaccines and Medical Biologicals (IVAC) on March 15.

Vietnam recruits volunteers for first phase of second COVID-19 vaccine
VOV.VN - The Institute of Vaccines and Medical Biologicals (IVAC) unveiled on the morning of March 5 that they have begun the process of receiving registrations from volunteers to participate in the initial phase of clinical trials for the second domestically-produced COVID-19 vaccine known as Covivac.
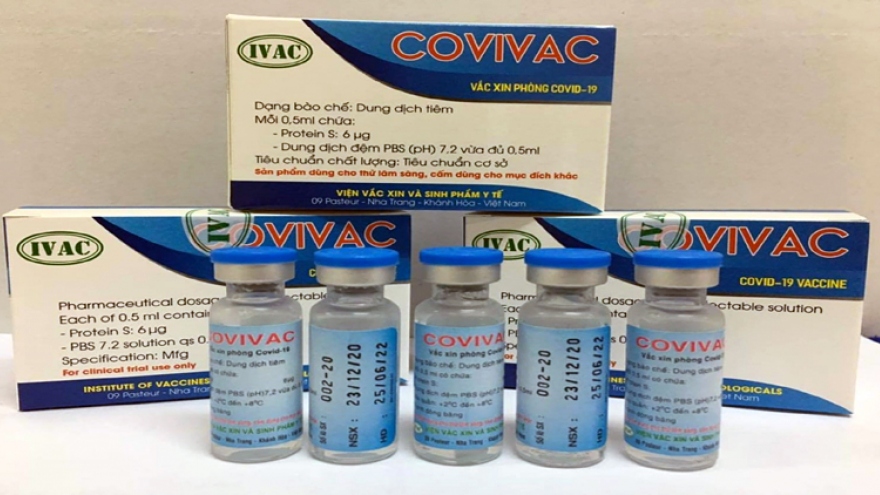
Second COVID-19 vaccine to begin human testing in Vietnam
VOV.VN - Covivac, a COVID-19 vaccine developed by the Institute of Vaccines and Medical Biology (Ivac), will be tested in healthy volunteers in Vietnam on March 3, Ivac has announced.
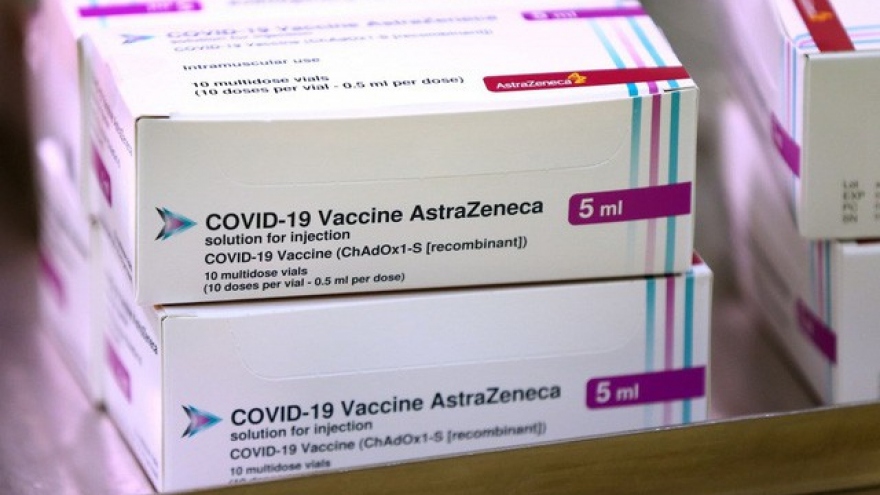
Questions arise over who will receive first COVID-19 vaccinations
VOV.VN - The initial batch of 204,000 doses of AstraZeneca’s novel coronavirus (COVID-19) vaccine are set to arrive in Vietnam over the coming days, with the focus now turning to which group of people will be prioritised to receive the first doses.

Vietnam tops ASEAN in Optimism Index amid COVID-19
Vietnam scored 62.4 points compared to 53.8 in Malaysia, 52.7 in Singapore, 52 in Thailand, and 49.6 in Indonesia.

Clinical trial research programme launched for second local COVID-19 vaccine
VOV.VN - A launching ceremony to mark the start of the clinical trial research scheme for the locally-produced novel coronavirus (COVID-19) vaccine, named COVIVAC, took place on January 21 at the Hanoi Medical University.
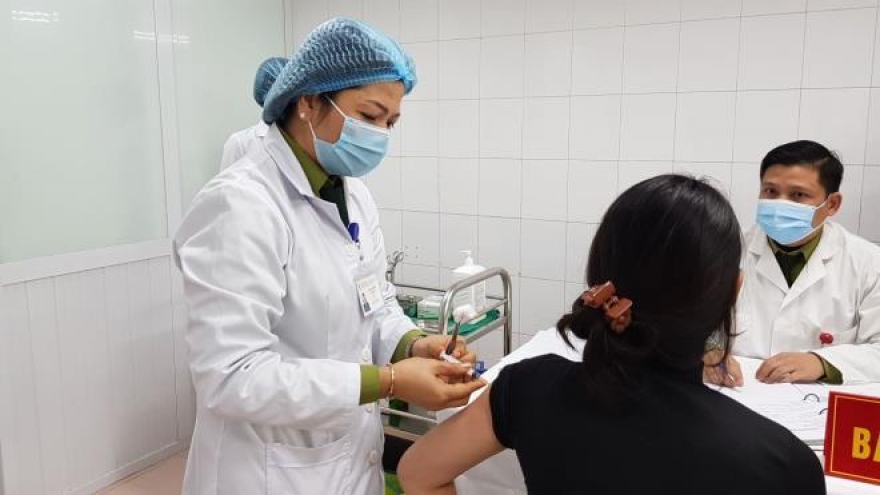
Second local COVID-19 vaccine to begin human trials in February
VOV.VN - The Ministry of Health has permitted the Institute of Vaccines and Medical Biologicals (IVAC) to carry out clinical human trials of its produced vaccine COVIVAC, starting in a couple of weeks.

A look at Made-in-Vietnam COVID-19 vaccines
VOV.VN - At present Vietnam has successfully produced two novel coronavirus (COVID-19) vaccines, namely Nanocovax and Covivac.
Vietnam to launch trials for second local COVID-19 vaccine in January
VOV.VN -The Ministry of Health has granted permission to the Nha Trang-based Institute of Vaccines and Biological Medical (IVAC) to carry out human clinical tests for Covivac, a locally-produced novel coronavirus (COVID-19) vaccine, on volunteers in January, two months ahead of schedule.





.jpg)



