Vietnam recalls infant formula suspected of cereulide contamination
VOV.VN - Vietnam’s Food Safety Authority (VFA) has issued an urgent warning and ordered the recall of five batches of infant formula products suspected of contamination with cereulide, a toxin produced by the bacterium Bacillus cereus that can pose serious health risks to children.
In a statement on January 27, the VFA, under the Ministry of Health, said it had received alerts from Hong Kong’s Centre for Food Safety (CFS) and Food Standards Australia New Zealand (FSANZ) regarding international recalls of several formula products.
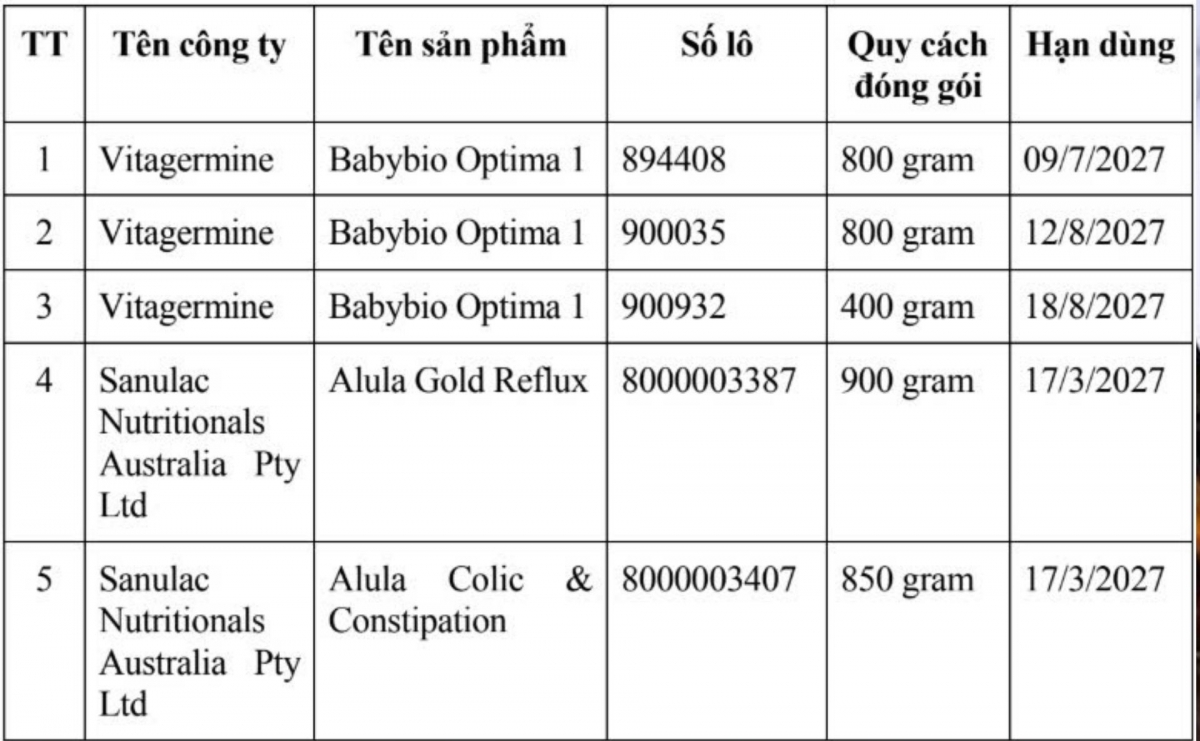
Three recalled products are Babybio Optima 1 infant formula manufactured by France-based Vitagermine, including batch numbers 894408 (800g, expiry July 9, 2027), 900035 (800g, expiry August 12, 2027) and 900932 (400g, expiry August 18, 2027).
Two additional products from Australia-based Sanulac Nutritionals Australia Pty Ltd are also affected: Alula Gold Reflux, batch 8000003387 (900g, expiry March 17, 2027) and Alula Colic & Constipation, batch 8000003407 (850g, expiry March 17, 2027).
Health experts said cereulide is heat-stable and cannot be destroyed by normal cooking or reheating, and may cause vomiting, diarrhea and liver damage, with infants and young children at highest risk.
Vietnamese authorities have instructed provincial health departments to review product registrations, work with importers and distributors to halt sales, notify consumers and conduct recalls in line with manufacturers’ recommendations. Agencies must report the quantities imported, sold and remaining in stock, along with proposed handling measures.
The VFA also said it has detected the recalled products still being advertised or sold on e-commerce platforms such as Shopee, Lazada and Ausmart, as well as on social media platforms including TikTok.
It has requested the Ministry of Industry and Trade’s E-commerce and Digital Economy Agency and the Ministry of Culture, Sports and Tourism’s relevant units to coordinate with platforms to remove listings and handle violations in accordance with regulations.
Parents and caregivers are advised to carefully check product names, batch numbers and expiry dates, stop using any affected products immediately and seek medical attention if children show symptoms such as vomiting, fatigue, abdominal pain or diarrhea.





