Over 60 volunteers to receive first jab of COVIVAC vaccine in second stage
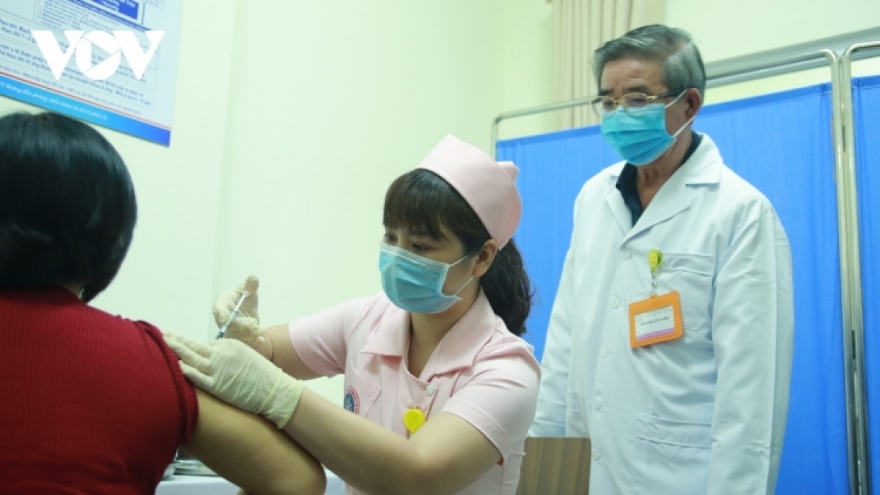
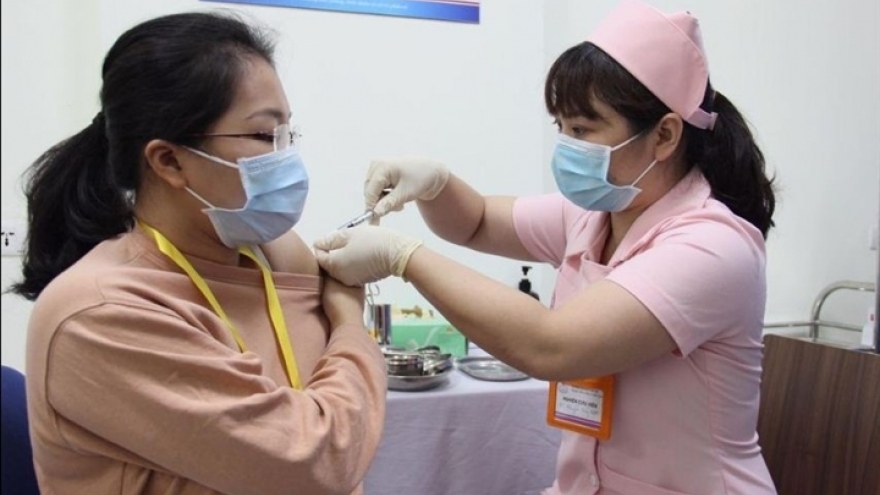
VOV.VN - More than 60 volunteers are poised to be given the first jab of the locally-produced COVIVAC vaccine on August 18 following it entering the second phase of clinical trials.

Assoc. Prof. Dr. Vu Dinh Thiem, director of the Center for Clinical Trials of the Central Institute of Hygiene and Epidemiology, said on August 12 that the research team has officially begun to screen and recruit suitable volunteers who will be injected with the first jab.
The trial injection programme is set to be deployed in Vu Thu district of Thai Binh province and will feature the participation of 375 volunteers.
These trialists will be divided into three groups, with members of the first group each receiving a three-mcg dose of COVIVAC, with the second group being given a six-mcg dose, and the third having a dose of the AstraZeneca vaccine.
Assoc. Prof. Thiem went on to reveal that through medical screening, a total of 187 volunteers have been deemed eligible for the clinical trials and have already signed the necessary consent forms to participate in the second phase.
In line with the schedule, the duration for the second phase of the COVIVAC vaccine will be from August 10 to September 20.
The research team will therefore conduct medical examinations and take blood samples from all participants for evaluation, before the research team then proposes the third phase of clinical trials to the Ministry of Health, he noted.
COVIVAC represents the country’s second COVID-19 vaccine to reach the clinical trial stage. The vaccine initially began the first phase of clinical trials involving 120 volunteers aged between 18 and 59 on March 15.
Furthermore, the locally-produced vaccine has been being researched and developed by the Institute of Vaccines and Medical Biologicals (IVAC) since May, 2020, representing a collaborative project with American universities and the PATH organisation.
Three manufacturing units, including IVAC-Vietnam, GPO-Thailand, and Butantan-Brazil have come together in order to jointly research and develop the vaccine through the technical support of PATH organisation.
The COVIVAC vaccine is a liquid vaccine which comes with or without adjuvants, without preservatives, whilst utlising the production technology of the Newcastle vector vaccine. It is based on the production technology used in chicken eggs with embryos, with this technology also being used to produce local seasonal flu vaccines.
The results of preclinical studies conducted in India, the United States, and Vietnam have so far proved the safety and efficacy of the domestic vaccine.