First phase of local COVIVAC human trials complete
VOV.VN - The nations has finalized the first stage of human trials on COVIVAC, the second locally-produced novel coronavirus (COVID-19) vaccine, with this phase featuring the participation of 120 volunteers.
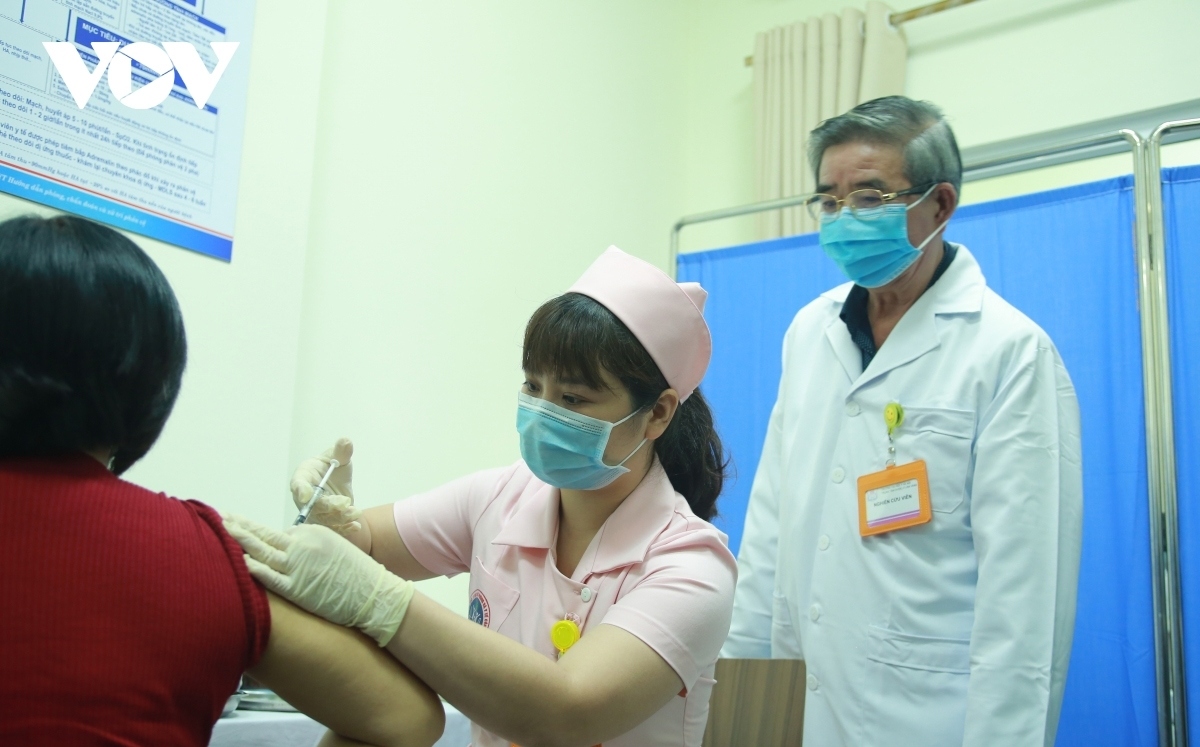
According to Vu Dinh Thiem, director of the Center for Clinical Trials under the National Institute of Hygiene and Epidemiology, a total of 120 volunteers received their second shot of the COVIVAC vaccine on May 15.
According to the schedule, Thiem stated that the research team will take samples from volunteers on May 29 for the purpose of carrying out an antibody test 14 days after being injected with their second shot.
Following this, the team will then send away all of the blood samples to be assessed in order to detect the antibody levels both before and after receiving the vaccine.
Providing that the vaccine shows that safety standards are met and the immunisation is capable of disease prevention based on approval granted by the Ministry of Health, then the second phase of clinical trials will be carried out at the Health Center of Vu Thu district in Thai Binh province.
The results of the second phase are set to be announced in either November or December.
This comes after COVIVAC, developed by the Institute of Vaccines and Medical Biologicals (IVAC), was proved to be effective against the new SARS-CoV strains detected in both the UK and South Africa on March 15.



