1,700 volunteers register for Nano Covax vaccine trials, third stage
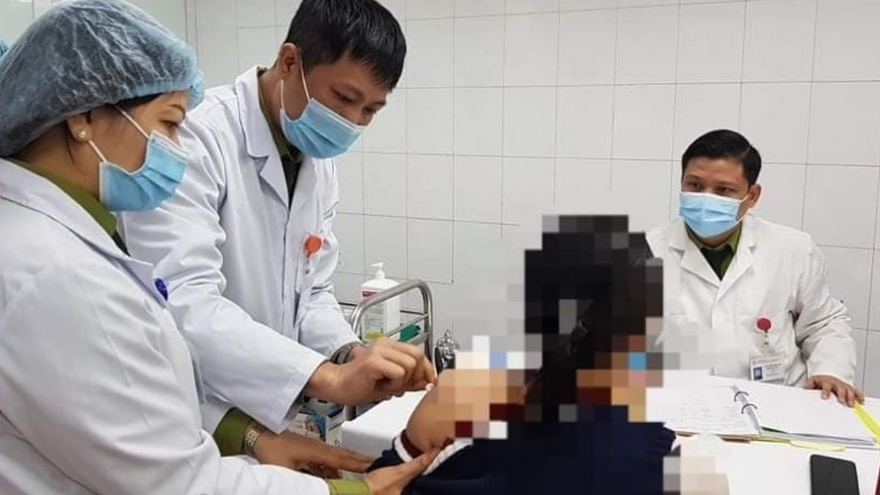
VOV.VN - As many as 1,700 volunteers have registered to participate in the third stage of human trials for the domestically-produced Nano Covax vaccine, with tests scheduled to begin in June.

According to Chu Van Men, director of the Military Medical University’s Centre for Clinical Trials and Bioequivalence, it is anticipated that the third phase will involve up to 15,000 people both in Vietnam and in other Asian countries hit by severe COVID-19 outbreaks.
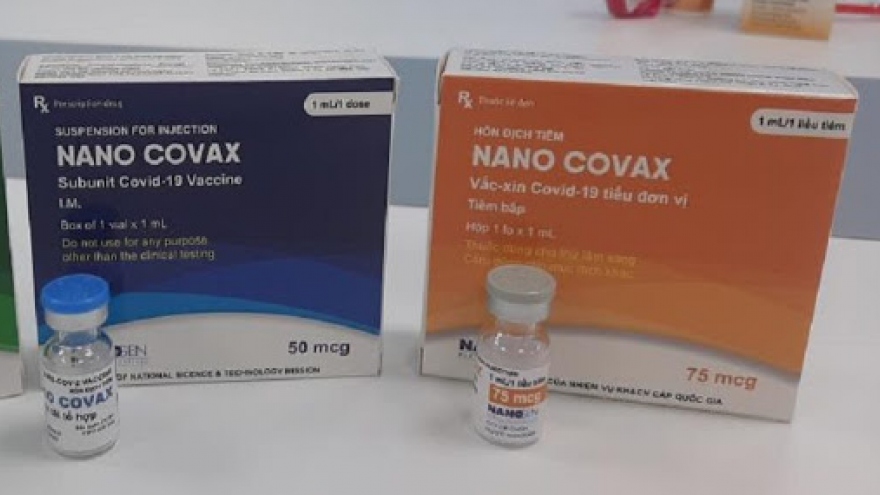
Volunteers will be administered with the only 25mcg dose of the vaccine, and the interval between the two shots is 28 days.
Men revealed that a total of 560 volunteers were administered with 25mcg, 50mcg, and 75mcg doses during the second phase of trials, and the initial assessment indicates that the Navo Covax vaccine is “relatively safe”.
All of the vaccinated volunteers developed antibodies against COVID-19 at different levels, he said.
He added that the vaccine has proved effective against mutated variants identified from the United Kingdom and South Africa. Scientists will asse the effectiveness of the virus against the Indian variant in the upcoming third phase.
In the event that the virus spreads very quickly in Vietnam, the vaccine manufacturer, Nanogen Pharmaceutical Biotechnology, will make a proposal to the Ministry of Health to use its product in early 2022.