Vietnam to produce Pfizer’s COVID-19 treatment pills
VOV.VN - Vietnamese pharmaceutical company Stellapharm has been licensed by international public health group Medicines Patent Pool (MPP) to produce Pfizer's COVID-19 treatment pills containing the active ingredient nirmatrelvir.
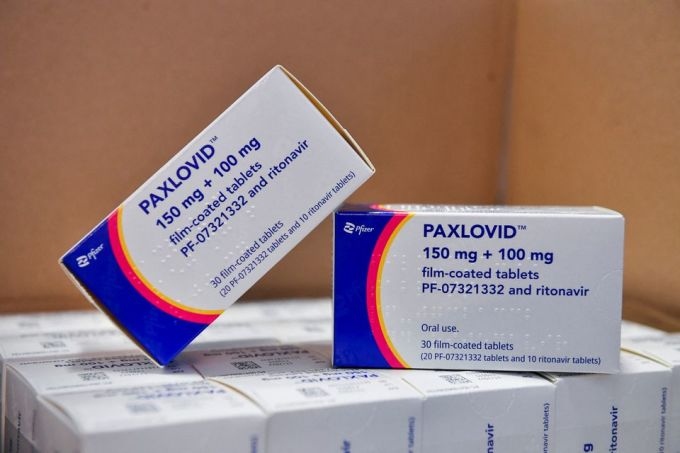
Stellapharm represents the only Vietnamese enterprise among 35 companies throughout 12 countries that have been franchised by MPP to produce such pills.
The 12 licensed franchisees include firms in Bangladesh, Brazil, China, Dominican Republic, Jordan, India, Israel, Mexico, Pakistan, Serbia, the Republic of Korea, and Vietnam.
Pfizer's drug, which is to be sold under the commercial name Paxlovid, was granted approval by the US Food and Drug Administration (FDA) in December, 2021.
Canada, the United Kingdom, and the European Medicines Agency (EMA) all allowed the drug to be used to treat patients from the age of 12, as well as old people suffering from underlying diseases such as obesity and diabetes.
The drug reduces the risk of hospitalisation or death by 88%, whilst it is also effective against the Omicron variant, according to health experts
Charles Gore, CEO of MPP, said nirmatrelvir is a new pharmaceutical product that requires significant manufacturing capacity, adding those franchisees have demonstrated their capability to meet MPP's requirements and produce products meeting international quality standards.
The franchise is expected to help bring the drug to countries that make up around 53% of the world’s population.
In December 2021, Pfizer, the owner of the nirmatrelvir drug patent, authorised MPP to review applications and sign contracts with pharmaceutical companies which are eligible to produce the drug in the global supply chain.
The Drug Administration of Vietnam under the Ministry of Health sent an official dispatch to the Vietnam Pharmaceutical Companies Association, requesting that local drug manufacturers be notified to submit their documents to become a franchise partner.
Earlier in January, Stellapharm was the only local pharmaceutical company licensed by MPP to produce drug maker MSD’s molnupiravir. The oral drug has been supplied to 105 low and middle-income countries.
At present, there are four types of antiviral active ingredients available to treat COVID-19 in Vietnam. Remdesivir is intravenously used for critically-ill patients, while molnupiravir, favipiravir, and nirmatrelvir are treatment pills for patients with mild symptoms.
At present, there are currently four types of antiviral active ingredients available to treat COVID-19, including remdesivir which is intravenously used for critically-ill patients, and three types of treatment pills, including molnupiravir, favipiravir, and nirmatrelvir for patients with mild symptoms.