Vietnam approves Remdesivir for COVID-19 treatment
VOV.VN - The Ministry of Health (MoH) has agreed to include Remdesivir, a broad-spectrum antiviral medication, in COVID-19 treatment protocol in Vietnam.
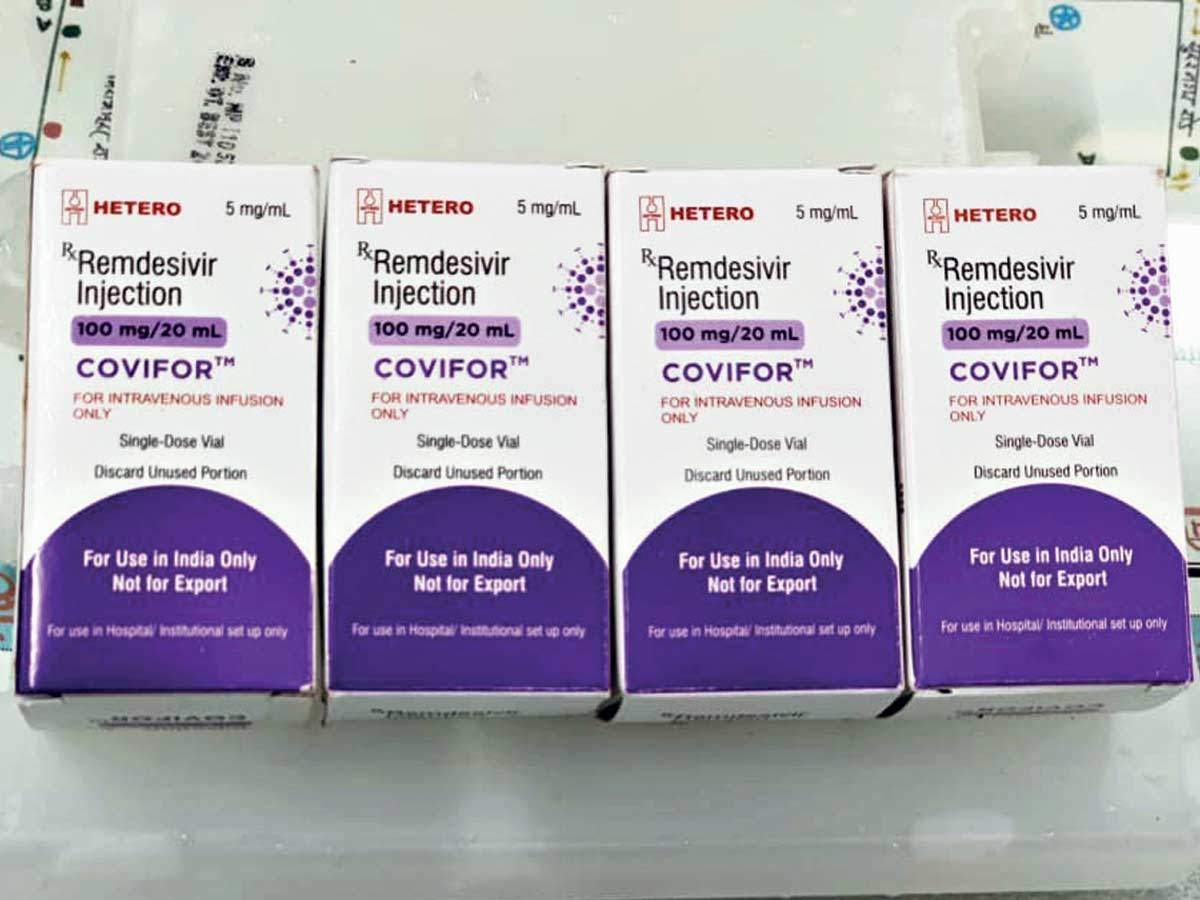
The decision was made following a meeting of the expert council of the MoH in Hanoi on August 6.
Remdesivir has been approved by the US Food and Drug Administration (FDA) to treat COVID-19 patients with moderate and severe symptoms. It has been used in more than 50 countries and territories worldwide such as the United States, the European Union, Japan, Singapore, and India.
However, Assoc. Prof. Dr. Luong Ngoc Khue, head of the Department of Health Examination and Treatment, noted Remdesivir supports COVID-19 treatment, it is not a special drug itself.
He also suggested the drug should be prescribed by a doctor and the patient should be monitored after use to evaluate the effectiveness of the drug.
India has recently agreed to deliver one million doses of Remdesivir to Vietnam, with half of the total purchased by Vingroup, a leading multisectoral conglomerate in Vietnam.
Vingroup earlier announced that it would donate 500,000 doses of Remdesivir to the Vietnamese government to support its ongoing fight against COVID-19.
The first batch of the drug arrived in HCM City on August 5 evening.
Dr. Khue said the entire amount of Remdesivir imported from India will be transferred to Ho Chi Minh City, southern provinces and some other epidemic hit localities.
Remdesivir, a much-sought-after antiviral drug, is manufactured by India’s Cipla Pharmaceutical Company with permission granted by US-based Gilead Sciences, the developer of the drug.